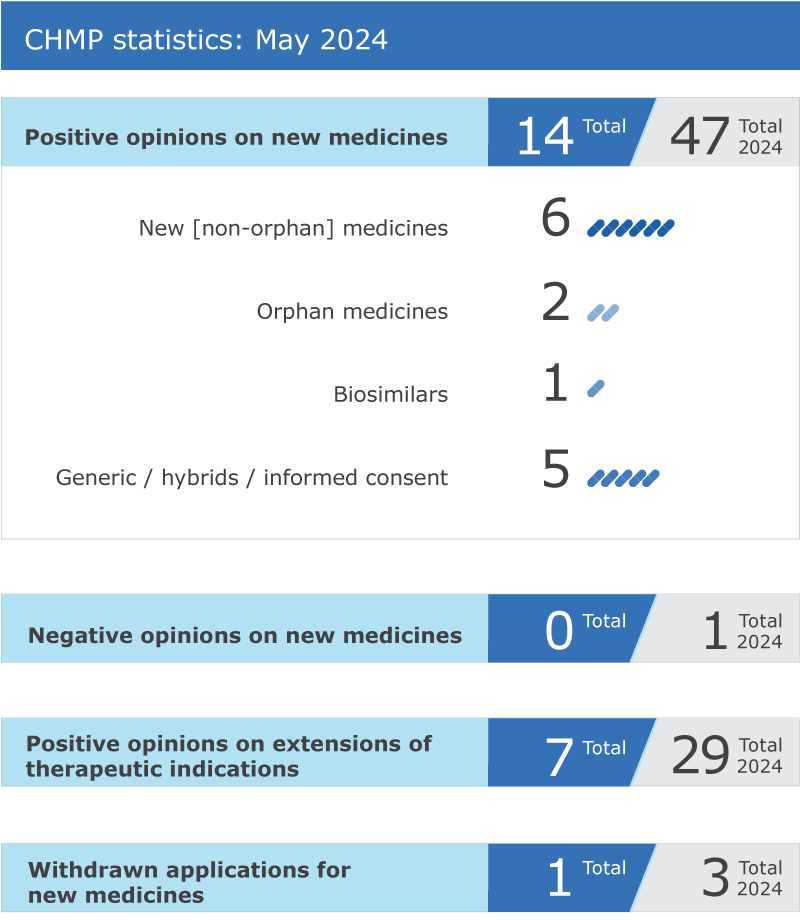
14 new drugs recommended for approval; seven other drugs are recommended for the extension of their therapeutic indications
The EMA’s Committee for Medicinal Products for Human Use (CHMP) recommended approval of 14 medicines at its May 2024 meeting.
The CHMP recommended granting marketing authorization in exceptional circumstances for Adzynma* (rADAMTS13), an enzyme replacement therapy indicated for the treatment of children and adults with congenital thrombotic thrombocytopenic purpura, a rare and life-threatening blood disorder characterized by blood clotting in small blood vessels throughout the body that can lead to organ damage and premature death. .
Akantior* (polyhexanide) received positive advice for the treatment of Acanthamoeba keratitis, a serious, progressive, sight-threatening corneal infection characterized by severe pain and photophobia. Acanthamoeba keratitis is a rare disease that mainly affects contact lens wearers.
The committee adopted a positive opinion for Leeks (sugemalimab) for the treatment of adults with metastatic non-small cell lung cancer.
A recommendation for conditional marketing authorization has been made for Durveqtix (fidanacogene elaparvovec), a new gene therapy treatment for hemophilia B, a rare inherited bleeding disorder. This medicine has been supported by the EMA’s PRIME (Priority Medicines) programme, which provides early and enhanced scientific and regulatory support for promising medicines that may address unmet medical needs. See more details in the news announcement in the grid below.
Fluenz (influenza vaccine (live attenuated, nasal)) has received a positive opinion for the prophylaxis of influenza in children and adolescents aged 24 months to less than 18 years.
The CHMP issued a positive opinion for GalliaPharm (germanium chloride (68Ge) / gallium chloride (68Ga)), to be used for radiolabeling various kits used for positron emission tomography (PET) imaging.
Drunk (chikungunya vaccine (live)), the first European Union (EU) vaccine to protect adults against disease caused by the Chikungunya virus transmitted to humans by infected mosquitoes, has received a positive opinion from the CHMP. Chikungunya is endemic in many (sub)tropical countries and causes recurrent epidemics. Due to climate change, it could also spread to regions previously spared. Ixchiq was supported by the PRIME program and was evaluated under the OPEN framework to promote global public health. See more details in the news announcement in the grid below.
The CHMP recommended granting a marketing authorization for From the Galogue (dasiglucagon) for the treatment of severe hypoglycemia in adults, adolescents and children aged six years and older with diabetes mellitus.
A biosimilar drug, Agitations (bevacizumab), received positive advice for the treatment of colon or rectal carcinoma, breast cancer, non-small cell lung cancer, renal cell cancer, epithelial ovarian cancer, tubal fallopian or primary peritoneum and carcinoma of the cervix.
Five generic drugs also received a positive opinion from the commission: Apexceline (paclitaxel) for the treatment of metastatic breast cancer; Dasatinib Health Accord (dasatinib) for the treatment of chronic myelogenous leukemia; Pomalidomide agreement (pomalidomide), Pomalidomide Krka (pomalidomide) and Pomalidomide Zentiva (pomalidomide) for the treatment of multiple myeloma.
Recommendations on therapeutic indication extensions for seven drugs
The commission recommended extensions of indication for seven medicines already authorized in the EU: Dupixent, Eliquis, Kinpeygo*, Livmarli*, Skyrizi, Tagrisso And Tevimbra.
Withdrawal of applications
An application for initial marketing authorization has been withdrawn. Kinharto was intended for the treatment of adult patients with symptomatic chronic heart failure and reduced ejection fraction when the heart muscle does not contract effectively.
The request for extension of therapeutic indication of Scene in adolescents with erythropoietic protoporphyria, a rare disease causing light intolerance, has been withdrawn.
Question and answer documents on these withdrawals are available in the grid below.
Result of the review
After reconsideration, the CHMP confirmed its initial recommendation to refuse the granting of a marketing authorization for Nezglyal. This medicine was intended to treat cerebral adrenoleukodystrophy, a genetic disease that damages the membrane covering nerve cells in the brain and spinal cord.
Other updates
Evaluation of the application for renewal of the marketing authorization of Translarna
The CHMP has relaunched the evaluation of an application for renewal of the conditional marketing authorization of The translators (ataluren), medicine authorized for the treatment of Duchenne muscular dystrophy.
In January 2024, the CHMP recommended not renewing the conditional marketing authorization of the medicine, based on its assessment of all available data.
The European Commission has now asked the Committee to examine in more detail whether the data available on Translarna are sufficiently comprehensive to allow conclusions to be drawn on the benefit-risk balance of the medicine, and whether actual additional data have been brought to the table. attention of the Commission during its decision-making process. may modify the negative result previously obtained by the CHMP.
Furthermore, following the appeal judgment of the Court of Justice of the European Union of March 14, 2024 in case C-291/22 P, the EMA has decided to convene a new scientific advisory group in neurology (SAG-N) for Translarna. The assessment is therefore reset at this stage of the initial renewal procedure. A SAG is a group of scientific experts called upon to answer specific questions asked by EMA committees during the evaluation of a medicine.
A revised recommendation from the CHMP on the renewal of Translarna’s marketing authorization is expected in the coming months. The marketing authorization for Translarna is currently still valid.
Result of the evaluation to expand the use of Valdoxan
CHMP finalizes its assessment of a request to extend the use of the antidepressant Valdoxan include treatment of adolescents with moderate to severe depression. Although the company decided not to pursue this use, the committee agreed that the product information be updated to include the results of the study submitted for the application. A question and answer document is available in the grid below.
Discussion paper on Creutzfeldt-Jakob disease
The CHMP adopted the revision of the discussion paper on Creutzfeldt-Jakob disease and medicinal products derived from plasma and urine. The main change brought about by this revision is that it is no longer recommended to exclude donors who spent at least one year in the UK between 1980 and 1996 from donating blood/plasma for fractionation. Cases of variant Creutzfeldt-Jakob disease in the UK have declined over the past two decades. The last known case in the UK was reported in 2016 and no transfusion-transmitted infections have been reported in the UK since 1999.
Agenda and minutes
The agenda for the May 2024 CHMP meeting is published on the EMA website. The minutes of the meeting will be published in the coming weeks.
CHMP Statistics
Key figures from the May 2024 CHMP meeting are shown in the graphic below.
*This product was designated an orphan drug during its development. Orphan designations are reviewed by the EMA’s Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows for maintaining the orphan status of the medicine and granting it ten years of use. commercial exclusivity.