
Researchers have shown, through experiments with high-energy lasers, that magnesium oxide is likely the first mineral to solidify in the formation of a super-Earth, which will have a crucial impact on evolution geophysics of these planets.
A new study reveals that magnesium oxide, a key mineral in planet formation, could be the first to solidify during the development of “super-Earth” exoplanets, with its behavior in extreme conditions significantly influencing the planetary development.
Scientists have for the first time observed how atoms in magnesium oxide transform and melt under extremely harsh conditions, providing new insights into this key mineral in Earth’s mantle, known to influence planet formation.
High-energy laser experiments, which subjected tiny crystals of the mineral to the type of heat and pressure found deep in the mantle of a rocky planet, suggest that this compound could be the first mineral to solidify from magma oceans to form “super-Earth” exoplanets. .
“Magnesium oxide may be the most important solid controlling the thermodynamics of young super-Earths,” said June Wicks, an assistant professor of Earth and planetary sciences at Johns Hopkins University who led the research. “If it had a very high melting temperature, it would be the first solid to crystallize when a hot, rocky planet begins to cool and its interior separates into a core and mantle.”
Implications for young planets
The results were recently published in 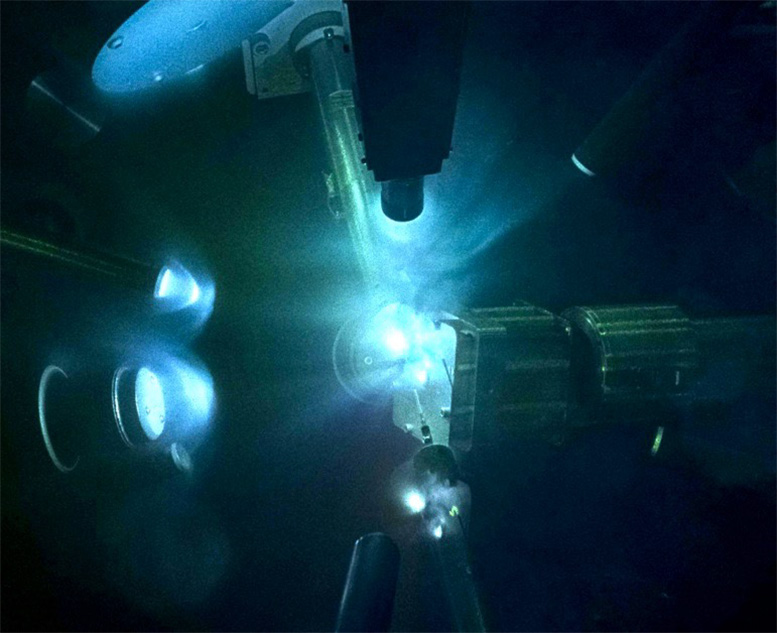
View of laser experiments on shock-compressed magnesium oxide (MgO) in the Laser Energy Laboratory chamber. High-power laser beams are used to compress MgO samples to pressures higher than those found at the center of the Earth. A secondary X-ray source is used to probe the crystal structure of MgO. The brightest regions emit luminous plasma on nanosecond time scales. Credit: June Wicks/Johns Hopkins University
Bigger than Earth but smaller than giants like
” data-gt-translate-attributes=”({“attribute”:”data-cmtooltip”, “format”:”html”})” tabindex=”0″ role=”link”>Neptune Or
” data-gt-translate-attributes=”({“attribute”:”data-cmtooltip”, “format”:”html”})” tabindex=”0″ role=”link”>Uranussuper-Earths are key targets in
” data-gt-translate-attributes=”({“attribute”:”data-cmtooltip”, “format”:”html”})” tabindex=”0″ role=”link”>exoplanet research because they are commonly found among other solar systems in the galaxy. Although the composition of these planets can vary from gas to ice to water, rocky super-Earths are expected to contain significant amounts of magnesium oxide which can influence the planet’s magnetic field, volcanism and other key geophysical parameters, as is the case on Earth, Wicks said. .
To mimic the extreme conditions this mineral might experience during planet formation, Wick’s team subjected small samples to ultra-high pressures using the Energy Laboratory’s Omega-EP laser facility laser from the University of Rochester. The scientists also took X-rays and recorded how these light rays bounced off the crystals to track how their atoms rearranged themselves in response to increasing pressures, specifically noting when they transformed from a solid to a liquid.
When squeezed extremely hard, the atoms of materials like magnesium oxide change their arrangement to withstand the crushing pressures. This is why the mineral changes from a rock salt “phase” resembling table salt to a different configuration like that of another salt called cesium chloride as pressure increases. This results in a transformation that can affect a mineral’s viscosity and impact a planet as it matures, Wicks said.
Stability of magnesium oxide at high pressure
The team’s results show that magnesium oxide can exist in its two phases at pressures ranging from 430 to 500 gigapascals and at temperatures of around 9,700 Kelvin, almost twice as hot as the surface of the sun. Experiments also show that the highest pressures the mineral can withstand before completely melting exceed 600 gigapascals, or about 600 times the pressure one would feel in the ocean’s deepest trenches.
“Magnesium oxide melts at a much higher temperature than any other material or mineral. Diamonds may be the hardest materials, but they are what will melt last,” Wicks said. “When it comes to extreme materials on young planets, the magnesium oxide will probably be solid, while anything else hanging out there in the mantle will be turned into a liquid.”
The study highlights the stability and simplicity of magnesium oxide under extreme pressures and could help scientists develop more precise theoretical models to probe key questions about how this and other minerals behave in rocky worlds like Earth, Wicks said.
“The study is a love letter to magnesium oxide, because it is astonishing that it has the melting point at the highest temperature we know of – at pressures beyond the center of the Earth – and it still behaves like regular salt,” Wicks said. “It’s just a simple, beautiful salt, even at these record pressures and temperatures.”
Reference: “B1-B2 Transition in Shock Compressed MgO” by June K. Wicks, Saransh Singh, Marius Millot, Dayne E. Fratanduono, Federica Coppari, Martin G. Gorman, Zixuan Ye, J. Ryan Rygg, Anirudh Hari, Jon H. Eggert, Thomas S. Duffy and Raymond F. Smith, June 7, 2024, Scientists progress.
DOI: 10.1126/sciadv.adk0306
Other authors are Saransh Singh, Marius Millot, Dayne E. Fratanduono, Federica Coppari, Martin G. Gorman, Jon H. Eggert and Raymond F. Smith of Lawrence Livermore National Laboratory; Zixuan Ye and Anirudh Hari of Johns Hopkins University; J. Ryan Rygg of the University of Rochester; and Thomas S. Duffy of
” data-gt-translate-attributes=”({“attribute”:”data-cmtooltip”, “format”:”html”})” tabindex=”0″ role=”link”>Princeton University.
This research was supported by NNSA through the National Laser User Facilities Program under Contract Nos. DE-NA0002154 and DE-NA0002720 and the LLNL Laboratory Directed Research and Development Program (Project No. 15-ERD-012). This work was carried out under the auspices of the U.S. Department of Energy by the Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344. The research was supported by the National Nuclear Security Administration through the National Laser Users’ Facility Program (Contract Nos. DE-NA0002154 and DE-NA0002720) and the LLNL Laboratory Directed Research and Development Program (Project No. ° 15-ERD-014, 17). -ERD-014 and 20-ERD-044).