A new drug to control liver disease has been given the green light.
The US Food and Drug Administration (FDA) has approved the drug from French manufacturer Ipsen. drug Iqirvo (elafibranor).
The medication, an 80 mg tablet administered orally once daily, is intended to treat an autoimmune cholestatic liver disease called primary biliary cholangitis (PBC).
What is CBP?
PBC is a disease in which the immune system attacks and destroys the small bile ducts in the liver.
IN ALZHEIMER’S BREAKTHROUGH, RESEARCHERS IDENTIFY ‘PROTECTIVE GENE’ THAT DELAYS DISEASE IN HIGH-RISK FAMILY
Without active bile ducts, acids can then leak into nearby tissues and cause liver damage or failure, according to the National Institutes of Health (NIH).
The US Food and Drug Administration (FDA) has approved the drug Iqirvo (elafibranor) from French manufacturer Ipsen. (iStock)
The disease usually involves chronic inflammation accompanied by a stagnant buildup of bile and toxins called cholestasis, which can lead to irreversible scarring of the liver and ultimately destroy the bile ducts.
Although PBC is considered a rare disease, it can often go unnoticed. health experts said.
“PBC is probably way underdiagnosed,” Dr. Douglas Dieterich, MD, director of the Liver Medicine Institute at Mount Sinai Health System in New York, told Fox News Digital.
CAN YOU BECOME SAVED WITHOUT DRINKING ALCOHOL? HERE’S HOW IT COULD HAPPEN
“Many people, mostly women, have elevated liver enzymes that can be easily diagnosed with a simple blood test called an AMA.”
Patients generally feel severe fatigue and severe itching called pruritus.
If the condition is left untreated or an individual does not respond to current treatments, it can lead to liver failure, the need for liver transplant or even premature death, according to experts.

PBC is a disease in which the immune system attacks and destroys the small bile ducts in the liver. Without active bile ducts, acids can then leak into nearby tissues and cause liver damage or failure. (iStock)
PBC is diagnosed with a blood test that measures liver enzymes.
A common test analyzes the patient’s alkaline phosphatase (ALP), an enzyme that helps detect liver or bone disease.
Another blood test to diagnose PBC measures antimitochondrial antibodies (AMA), which are positive in about 95% of patients with this disease, according to several liver experts.
Patient welcomes more treatment options
A New York patient with PBC told Fox News Digital that she didn’t know she had liver disease until she died. primary care doctor had routine blood tests done and noticed his liver enzymes were elevated.
Meredith S., who withheld her last name for privacy reasons, was referred to a hepatologist, whom she credits with saving her life.
“It’s painful to know that your body is fighting against itself and you don’t know how to stop it.”
“I felt tired, but I attributed that to work and studying at school,” she told Fox News Digital.
“I was completely surprised to learn that I had liver disease and learned that it was PBC.”
She continued: “My doctor did a liver biopsy and I had significant liver scarring in my 30s, even though I didn’t drink alcohol.”

Patients have expressed relief that they have another treatment option for liver disease. (iStock)
Meredith S. says she’s glad there are more treatment options available and hopes for more awareness and research into PBC.
“It’s painful to know that your body is fighting on its own and you don’t know how to stop it,” she told Fox News Digital.
Addressing an “unmet need”
Dieterich of New York, who is also a professor of medicine at the Icahn School of Medicine at Mount Sinai, told Fox News Digital that this newly approved drug “is a giant step forward in the treatment of PBC.”
He noted: “Until now, there was only one drug available…Now there are two. »
STUDY DISCOVERS “TRIGGER GENE” IN IBD AS RESEARCHERS SEEK DRUGS TO PREVENT BOWEL DISEASE
The existing drug, ursodeoxycholic acid (UDCA) – commonly known as ursodial or “urso” – is a natural bile acid that has been used to treat liver disease for decades.
Recently approved Iqirvo (elafibranor) is intended for use in combination with UDCA in patients who do not respond to the first drug alone, or may be used alone in people who cannot tolerate UDCA.
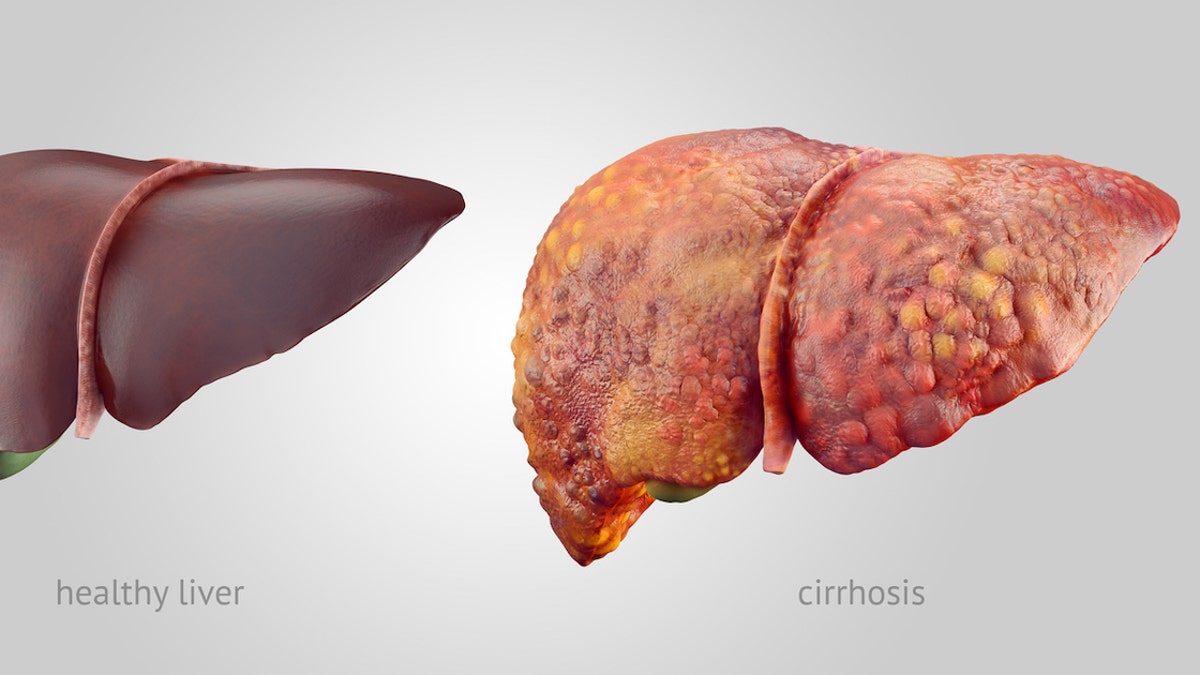
The disease usually involves chronic inflammation accompanied by a stagnant buildup of bile and toxins called cholestasis, which can lead to irreversible scarring of the liver and ultimately destroy the bile ducts. (iStock)
“For a significant number of people living with PBC, available treatments do not control the disease and can exacerbate PBC symptoms,” said Christelle Huguet, executive vice president and head of research and development at Ipsen, in a press release.
“Iqirvo demonstrated statistically significant improvements in biochemical response compared to UDCA alone. Iqirvo is therefore a much-needed treatment option and the first new drug for PBC in almost a decade.
Primary biliary cholangitis affects approximately 100,000 people in the United States
The accelerated approval of Iqirvo was based on the positive results of the ELATIVE phase III trialwhich showed reduced levels of the enzyme alkaline phosphatase, which is elevated in people with liver disease.
The study, published in the New England Journal of Medicine, included 161 patients with PBC who did not respond adequately to treatment with UDCA or could not tolerate the drug.
CLICK HERE TO SUBSCRIBE TO OUR HEALTH NEWSLETTER
Researchers found that 51% of patients who received elafibranor had a biochemical response, compared to only 4% who received a placebo.
After 52 weeks, patients treated with elafibranor had normalized liver enzymes compared to 15% of patients in the placebo group.

The study, published in the New England Journal of Medicine, included 161 patients with PBC. (iStock)
“Data from the pivotal Phase III ELATIVE clinical trial demonstrated that Iqirvo is an effective second-line treatment for patients with PBC, with favorable benefit and risk data,” said Dr. Kris Kowdley , principal investigator of the ELATIVE study and director of the Liver Institute. Northwest, Washington, said in a press release.
CLICK HERE TO GET THE FOX NEWS APP
“The approval of Iqirvo will allow healthcare providers in the United States to address an unmet need, with the potential to significantly reduce ALP levels in our PBC patients,” he said. added.
Continued approval is contingent on further studies demonstrating improved survival or prevention of liver breakdown, the FDA report said.
Potential side effects and limitations
Some reported side effects of Iqirvo included muscle painrhabdomyolysis, myopathy, fractures, weight gain and drug-induced liver injury, according to the FDA report.

Some reported side effects of Iqirvo included muscle pain, rhabdomyolysis, myopathy, fractures, weight gain and drug-induced liver injury, according to the FDA report. (iStock)
The potential risk to the fetus was also noted. pregnant patientsbased on data from animal studies.
The FDA has warned health care providers to ensure patients are not pregnant before starting treatment.
Iqirvo is also not recommended in patients with advanced cirrhosis.
For more health articles, visit www.foxnews/health
Primary biliary cholangitis affects approximately 100,000 people in the United States, according to the pharmaceutical company Ipsen.
It is a lifelong condition that can lead to liver failure if left untreated, experts say.