By

Alzheimer’s disease, a progressive neurodegenerative disease that affects approximately 55 million people worldwide, causes severe cognitive decline and memory loss. Researchers at the Okinawa Institute of Science and Technology have developed a synthetic peptide, PHDP5, that targets early-stage Alzheimer’s disease by ensuring the availability of dynamin for vesicle recycling in neurons, demonstrating a significant restoration of memory and learning functions in transgenic mice.
A new treatment has been shown to effectively combat cognitive decline in mice with Alzheimer’s disease.
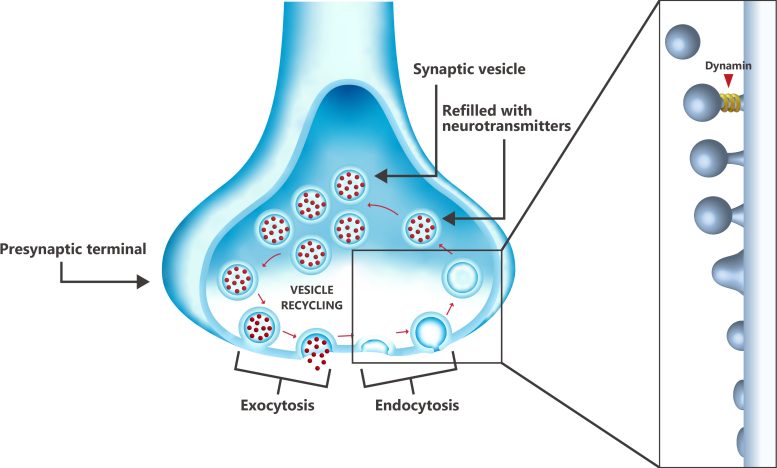
Recycling of vesicles into the presynaptic terminal at one end of a neuron, showing the role of dynamin during the final step of endocytosis (membrane retrieval), where the protein cleaves the vesicle from the cell membrane. The vesicle is then filled with neurotransmitters and transported back to the cell membrane release site, where the neurotransmitters are released and the vesicle is recycled. Credit: Kaori Serakaki/OIST
Saving dynamin
The health of brain synapses is a central factor in Alzheimer’s disease. Synapses are the junctions between neurons in the brain, where information is transmitted from one neuron to another via chemical neurotransmitters enclosed in synaptic vesicles. These vesicles must be constantly recycled to ensure a constant supply, and an essential step in the vesicle recycling process is membrane scavenging (endocytosis) by the protein dynamin, which “cuts” the vesicle from the cell membrane. Dynamin is available in all neurons, either freely or bound to the microtubules which constitute the cytoskeleton of the cells.
The key antagonist here is the tau protein, which under normal circumstances participates in the stabilization of microtubules. However, in the early stages of Alzheimer’s disease, tau begins to dissociate from microtubules. Being freely available, tau over-assembles new microtubules, effectively sucking dynamin out of the cell, making it unavailable for the final step of endocytosis. As Alzheimer’s disease progresses, accumulated tau aggregates into neurofibrillary tangles, which are the hallmark of the disease. By the time these tangles appear on brain scans, it is often too late to treat the condition.
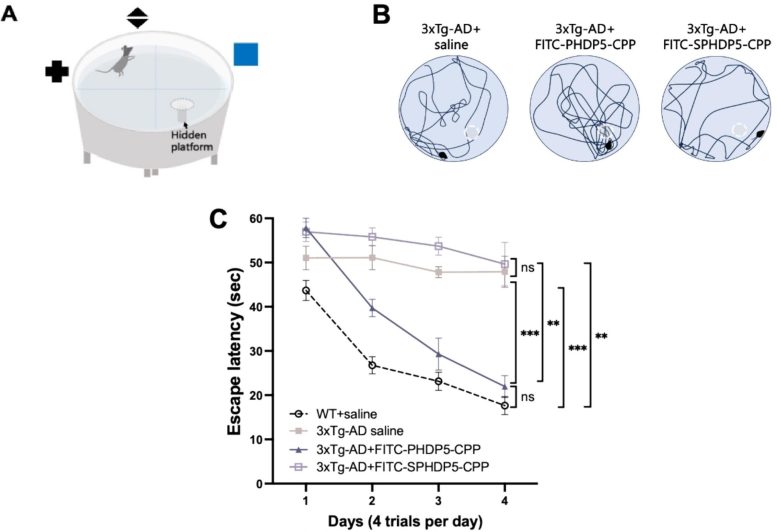
Some of the main conclusions of the article. SPHDP5 is a scrambled peptide with no therapeutic effect, used as a control. A) shows the experimental setup with a Morris water maze, in which a mouse is placed in a water bath and trained to find a hidden platform using visual cues. B) are representative illustrations of the mice’s swimming paths to the hidden platform (white dotted line). C) shows the effect of intranasal administration of PHDP5 over time – notice how the curves of healthy mice (dotted black line) and transgenic mice treated with PHDP5 (gray line with triangles) are very similar. Credit: Chang et al.
OIST researchers focused specifically on the dynamin-microtubule interaction and have already proven the positive effects of inhibiting this interaction in vitro using the synthetic peptide PHDP5. Dr. Zacharie Taoufiq, currently in the Synapse Biology Unit at OIST and second author of the paper, explains: “By preventing the interaction between dynamin and microtubules, PHDP5 ensures that dynamin is available for endocytosis of vesicles during recycling, which can restore lost communication. between neurons inside synapses at an early stage.
Using transgenic mice, researchers have now demonstrated the same repair effect in vivo. “We were pleased to find that PHDP5 significantly rescued learning and memory deficits in mice,” says Dr. Chang. “This success highlights the potential of targeting the dynamin-microtubule interaction as a therapeutic strategy for Alzheimer’s disease. »
Because PHDP5 generally inhibits dynamin-microtubule interactions, the researchers modified the peptide to include a cell-penetrating peptide, which allows the treatment to be delivered through the nasal cavity where the blood-brain barrier is not completely developed and which is close to the memory center of the brain, the hippocampus. In this way, the peptide would be delivered to the hippocampus at a higher concentration than through other delivery methods, while minimizing potential side effects elsewhere in the body.
From molecules and labyrinths to viable treatments
Provided that synapses are treated with PHDP5 at a relatively early stage, the damage caused by the rampant dynamin-microtubule interaction can be reversed to the point that treated transgenic mice have learning and memory abilities comparable to mice. healthy. Although the peptide cannot cure Alzheimer’s disease, inhibition of dynamin-microtubule interaction significantly delays cognitive decline, to the point that it may not affect healthy people over a lifetime normal.
Emboldened by these results, the research team, now led by Dr. Taoufiq and composed of specialists from different OIST units, continues its work on the treatment. Dr Taoufiq, based at the Synapse Biology Unit, is working to improve the peptide itself and its functions in vivo. “We want to increase the amount of PHDP5 in the brain to get better effects, while minimizing side effects,” he explains. Meanwhile, Dr Chang, based at the Neural Computing Unit, is working on introducing AI into the search for additional and more robust data: “We are using the different areas of expertise at OIST to improve our research. »
At the same time, the team is working with the OIST Innovation division to advance the peptide through the production pipeline. “We now want to involve pharmaceutical companies,” explains Dr Taoufiq. “They have the necessary pharmacology expertise and human testing capacity to turn our peptide into a viable treatment. »
Even if the journey between research and medicine is sadly long, taking on average 20 years between publication on paper and prescription, researchers remain very enthusiastic. As Dr. Chang says: “The coronavirus vaccine has shown us that treatments can be developed quickly, without sacrificing scientific rigor or safety. We don’t expect it to happen this quickly, but we know that governments – particularly in Japan – want to tackle Alzheimer’s disease, which affects so many people. And now we have learned that it is possible to effectively reverse cognitive decline if it is treated early.
Commentary from OIST Professor Emeritus Tomoyuki Takahashi
Now retired from OIST, Professor Takahashi initiated the project and led it until the unit closed. “In this study, as well as in the previous one, we clarified the pathological significance of dynamin-microtubule (MT) interaction in Alzheimer’s disease (AD), by which synaptic functions are significantly impaired. The dynamin-MT inhibitor, PHDP5, rescues synaptic dysfunction caused by tau accumulation in brain slices and can return learning and memory deficits to normal levels in AD transgenic mouse models. This in vivo effect is robust since it is reproducible in double-blind tests and consistent in two types of mouse models. Clearly, the crucial next step is to put PHDP5 through Phase 1-4 testing of AD therapeutic trials, which would be better done by pharmaceutical companies. We very much hope that our peptide can pass the tests and reach AD patients without much delay and rescue their cognitive symptoms, which are the main concern of patients and their families.
Reference: “The microtubule and dynamin binding inhibitor peptide PHDP5 rescues spatial learning and memory deficits in mouse models of Alzheimer’s disease” by Chia-Jung Chang, Zacharie Taoufiq, Hiroshi Yamada, Kohji Takei, Takami Tomiyama, Tomohiro Umeda, Tetsuya Hori and Tomoyuki Takahashi, May 6, 2024, Brain research.
DOI: 10.1016/j.brainres.2024.148987
The study was funded by the Okinawa Institute of Science and Technology.