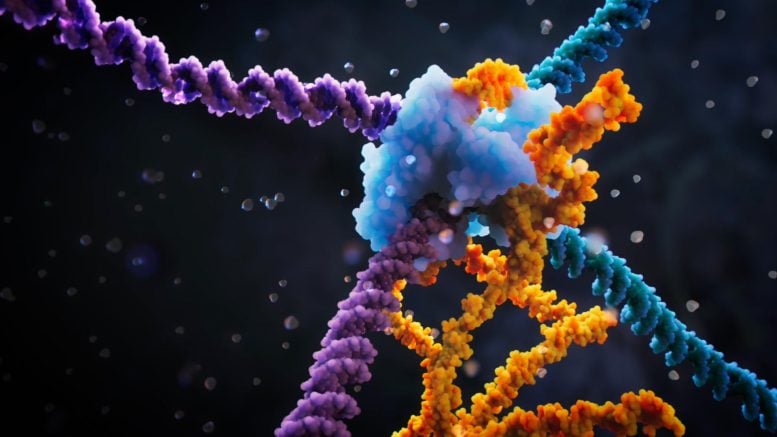
Visualization of the bridge recombinase mechanism. Credit: Visual Science
Arc Institute scientists have discovered the bridge recombinase mechanism, a revolutionary tool that enables complete programming
” data-gt-translate-attributes=”({“attribute”:”data-cmtooltip”, “format”:”html”})” tabindex=”0″ role=”link”>DNA rearrangements.
Their conclusions, detailed in a recent report Nature publication, is the first DNA recombinase that uses a non-coding gene
” data-gt-translate-attributes=”({“attribute”:”data-cmtooltip”, “format”:”html”})” tabindex=”0″ role=”link”>ARN for the specific selection of target and donor DNA molecule sequences. This RNA bridge is programmable, allowing the user to specify any desired genomic target sequence and any donor DNA molecule to be inserted.
The research was developed in collaboration with the laboratories of Silvana Konermann, principal investigator of the Arc Institute and assistant professor of biochemistry at Stanford University, and Hiroshi Nishimasu, professor of structural biology at the University of Tokyo.
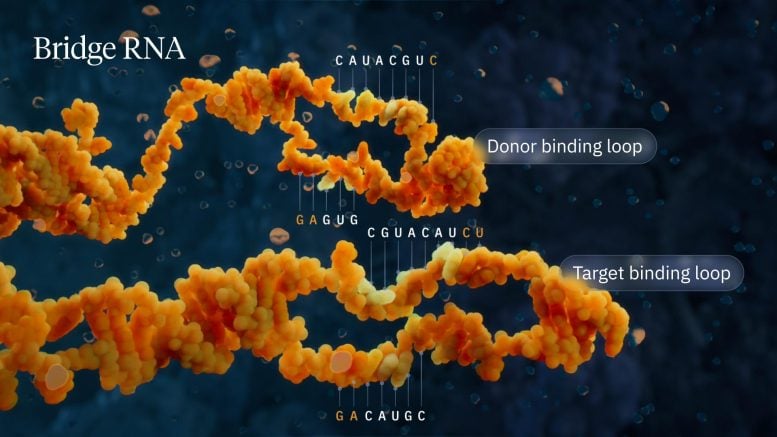
Visualization of the bridge recombinase mechanism highlighting the donor and target binding loops. Credit: Visual Science
A New Era of Genetic Programming
“The RNA bridge system is a fundamentally new mechanism for biological programming,” said Dr. Patrick Hsu, lead author of the study and principal investigator at the Arc Institute and
” data-gt-translate-attributes=”({“attribute”:”data-cmtooltip”, “format”:”html”})” tabindex=”0″ role=”link”>University of California, Berkeley Assistant Professor of Bioengineering. “Bridge recombination can universally modify genetic material through insertion, excision, inversion of specific sequences and more, enabling text processing for the living genome beyond CRISPR.”
The bridge recombination system arose from insertion sequence 110 (IS110) elements, one of countless types of transposable elements—or “jumping genes”—that cut and paste to move within and between microbial genomes. Transposable elements are present in all forms of life and have evolved to become professional DNA-manipulating machines for survival. IS110 elements are very small, consisting only of a gene encoding the enzyme recombinase, plus flanking DNA segments that have remained a mystery until now.
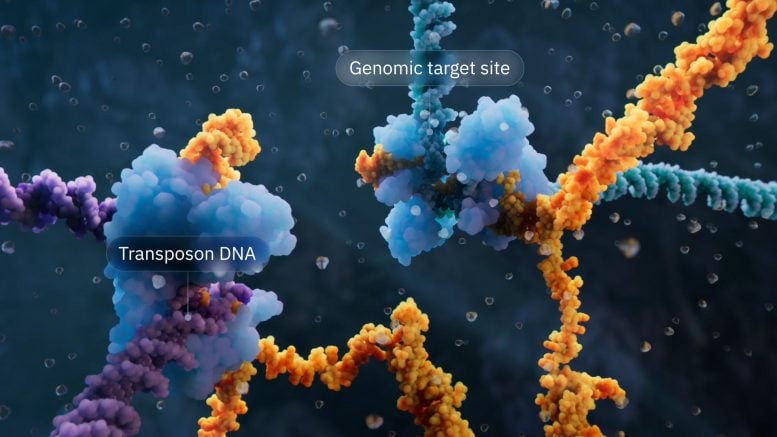
Visualization of the bridge recombinase mechanism highlighting the transposon DNA and the genomic target site. Credit: Visual Science
Advanced RNA bridge mechanism
Hsu’s lab discovered that when IS110 excises itself from a genome, the noncoding ends of the DNA join together to produce an RNA molecule—the bridging RNA—that folds into two loops. One loop binds to the IS110 element itself, while the other loop binds to the target DNA where the element will be inserted. The bridging RNA is the first example of a bispecific guide molecule, specifying the sequence of the target DNA and the donor DNA through base-pairing interactions.
A team of researchers at the Arc Institute has discovered the bridge recombinase mechanism, a precise and powerful tool to recombine and rearrange DNA in a programmable way. Going far beyond programmable genetic scissors like CRISPR, the bridge recombinase mechanism allows scientists to specify not only the target DNA to edit, but also the donor material to recognize, so they can insert new functional genetic material, cut out defective DNA, or reverse two sequences of interest. Learn more in this short video visualizing key aspects of the bridge recombination mechanism. Credit: Visual Science
Each loop of the RNA bridge is independently programmable, allowing researchers to mix and match any target and donor DNA sequence of interest. This means the system can go well beyond its natural role of inserting the IS110 element itself, instead allowing the insertion of any desirable genetic cargo—such as a functional copy of a defective disease-causing gene—into any genomic location. In this work, the team demonstrated an insertion efficiency of more than 60% of a desired gene into E. coli with over 94% specificity for the correct genomic location.
“These programmable bridge RNAs distinguish IS110 from other known recombinases, which do not have an RNA component and cannot be programmed,” said Nick Perry, co-senior author and graduate student in bioengineering at the University of California, Berkeley. “It’s as if the bridge RNA were a universal power adapter that would make IS110 compatible with any outlet.”

Patrick Hsu, Nick Perry, and Matt Durrant discuss the newly discovered bridge recombinase mechanism. Credit: Ray Rudolph
Collaborative research and future implications
The Hsu lab’s discovery is complemented by their collaboration with the lab of Dr. Hiroshi Nishimasu at the University of Tokyo, also published June 26 in NatureNishimasu’s lab used cryo-electron microscopy to determine the molecular structures of the RNA-bridge recombinase complex bound to the target and donor DNA, progressing sequentially through key steps in the recombination process.

Januka Athukoralage, Nicholas Perry, Silvana Konermann, Matthew Durrant, Patrick Hsu, James Pai and Aditya Jangid. Credit: Ray Rudolph
With further research and development, the bridge mechanism promises to pave the way for a third generation of RNA-guided systems, going beyond the DNA and RNA cutting mechanisms of CRISPR and RNA interference (RNAi) to provide a unified mechanism for programmable DNA rearrangements. Essential for the further development of the bridge recombination system for mammalian genome design, the bridge recombinase joins the two DNA strands without releasing the cut DNA fragments, circumventing a key limitation of current state-of-the-art genome editing technologies.
“The bridge recombination mechanism solves some of the most fundamental challenges facing other genome editing methods,” said Matthew Durrant, co-lead researcher and senior scientist at Arc. “The ability to programmatically rearrange two DNA molecules opens the door to advances in genome design.”
The references:
“Bridge RNAs Direct Programmable Recombination of Target and Donor DNA” by Matthew G. Durrant, Nicholas T. Perry, James J. Pai, Aditya R. Jangid, Januka S. Athukoralage, Masahiro Hiraizumi, John P. McSpedon, April Pawluk, Hiroshi Nishimasu, Silvana Konermann, and Patrick D. Hsu, June 26, 2024, Nature.
DOI: 10.1038/s41586-024-07552-4
“Structural Mechanism of Bridging RNA-Guided Recombination” by Masahiro Hiraizumi, Nicholas T. Perry, Matthew G. Durrant, Teppei Soma, Naoto Nagahata, Sae Okazaki, Januka S. Athukoralage, Yukari Isayama, James J. Pai, April Pawluk, Silvana Konermann, Keitaro Yamashita, Patrick D. Hsu, and Hiroshi Nishimasu, June 26, 2024, Nature.
DOI: 10.1038/s41586-024-07570-2