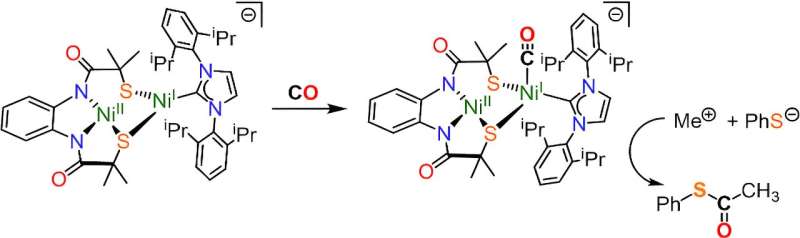
When the ACS enzyme is exposed to carbon monoxide, it produces acetyl Co-A, a molecule essential to all living things. The team’s model active site was able to accurately reproduce this phenomenon. Credit: Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c06241
Scientists at King’s College London have recreated the active site of acetyl-CoA synthase, an enzyme involved in capturing carbon from the atmosphere. This research, conducted in collaboration with Imperial College London, advances our understanding of this important enzyme and offers a potential new solution for capturing CO2 of the atmosphere in the fight against climate change.
Led by Dr Rebecca Musgrave from the Department of Chemistry and Dr Daniel Wilson from UCL, the team successfully recreated an active site – the site where chemical reactions take place – of the enzyme acetyl-CoA synthase (ACS).
ACS transforms CO2 into acetyl coenzyme-A, an essential molecule used by living beings. Their results are published in Journal of the American Chemical Society.
ACS is best known for its role in the acetic acid cycle or Krebs cycle, a series of chemical reactions in living things in which acetic acid is oxidized to produce energy. It is therefore essential for storing and releasing energy and for capturing CO2 from the atmosphere and stores it as carbon.
The team’s new model was able to replicate this chemical reaction in the lab, capturing atmospheric carbon and storing it as acetyl coenzyme-A.
Enzymes are proteins that function as biological catalysts by speeding up chemical reactions. As such, they perform vital functions in nature, including in human biology.
The chemical pathways created by enzymes have evolved over billions of years into large, complex biological systems and are therefore very difficult to study and reproduce in the laboratory. Scientists often recreate smaller molecular versions of enzymes (models of the “active site”) in the laboratory to study them.
The ACS enzyme is present in bacteria and some single-celled organisms and functions without oxygen, building complex organic molecules from carbon dioxide and hydrogen. Although attempts have been made to model the enzyme’s active site in the laboratory, they have not been able to accurately reproduce the shape and electronic environment of the active site for capturing carbon.
Dr Daniel Wilson, lead researcher from UCL, said: “Scientists have been studying the ACS enzyme for decades, but it has been difficult to decipher the mechanism that produces acetyl coenzyme-A in the enzyme’s active site. In our study, we present a model of the active site – a molecular cluster with two nickel atoms – that mimics the shape and size with remarkable similarity to the active site of the ACS enzyme.
“Interestingly, exposing our model to carbon monoxide allowed for successful synthesis, mimicking how the ACS enzyme produces acetyl Co-A in nature.”
Working with Dr Maxie Roessler from Imperial College, the team used a technique called electron paramagnetic spectroscopy to study the steps involved and believe the results will provide valuable insights for scientists studying the ACS enzyme and other enzymes linked to atmospheric carbon fixation, or carbon capture.
Dr Rebecca Musgrave said: “Our new model paves the way for a better understanding of how this reaction works. By studying the different stages of the reaction using electron paramagnetic resonance spectroscopy and other techniques, we can use what we learn to inform the design of artificial catalysts for industrial use.
“This could be applied to many areas, including new methods of capturing CO2 from the atmosphere and use it as a feedstock to produce carbon-based chemicals such as biofuels for cars or pharmaceuticals.
The researchers also hope that those working in the field of enzyme spectroscopy (the field of studying enzymes) will be able to take their new model and adapt it for use in their own studies.
Dr Musgrave said: “Enzymes found in nature achieve these incredible transformations quickly and efficiently, in ways that are very difficult to replicate in the laboratory. Our new model brings us one step closer to understanding how these biological systems do this so well, so that we can design industrial-scale catalysts to replicate nature’s transformative capacity and tackle key societal challenges such as climate change.”
More information:
Daniel WN Wilson et al., Mixed valence {Ni2+In1+} Clusters as models of acetyl coenzyme A synthase intermediates, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c06241
Provided by King’s College London
Quote: Scientists Replicate Enzyme That Captures Carbon (2024, July 18) Retrieved July 19, 2024 from https://phys.org/news/2024-07-scientists-replicate-enzyme-captures-carbon.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.