
Researchers have identified a biological mechanism involving the molecule KIBRA that explains the long-term stability of memories, shedding light on potential treatments for memory-related disorders.
Pioneering study reveals ‘molecular glue’ essential for memory formation and stabilization.
New research identifies the molecule KIBRA as a critical “glue” for stabilizing long-term memories by maintaining synaptic strength, providing insights into how memory persists despite ongoing cellular changes.
Whether it’s a first visit to the zoo or learning to ride a bike, we have memories from our childhood that last into adulthood. But what explains how these memories last almost a lifetime?
A new study in the journal 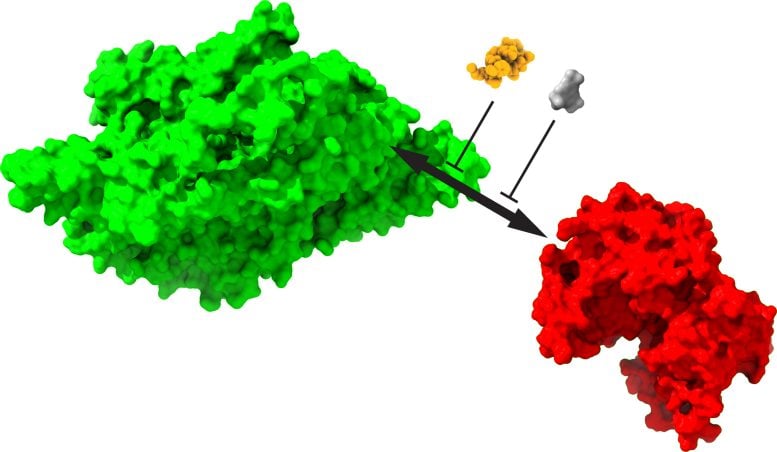
Memories are stored through the interaction of two proteins: a structural protein, KIBRA (green), which acts as a persistent synaptic tag, and a synapse-strengthening enzyme, protein kinase Mzeta (red). Drugs that disrupt the interaction between memories (other colors) erase long-term memories and pre-established old memories. Credit: Changchi Hsieh, Ph.D.
In a study in laboratory mice, the scientists focused on the role of KIBRA, or kidney and brain protein, whose human genetic variants are associated with both good and poor memory. They focused on KIBRA’s interactions with other molecules critical to memory formation, in this case, protein kinase Mzeta (PKMzeta). This enzyme is the most crucial molecule known to strengthen normal mammalian synapses, but it degrades after a few days.
Their experiments reveal that KIBRA is the “missing link” in long-term memories, serving as a “persistent synaptic tag,” or glue, that sticks to strong synapses and PKMzeta while avoiding weak synapses.
Memory retention mechanisms
“During memory formation, the synapses involved in that formation are activated and KIBRA is selectively positioned at those synapses,” says Sacktor, professor of physiology, pharmacology, anesthesiology and neurology at SUNY Downstate. “PKMzeta then attaches to the KIBRA synaptic tag and keeps those synapses strong. This allows the synapses to stick to the newly formed KIBRA, thereby attracting more newly formed PKMzeta.”
More specifically, their experiences in the Scientific progress documents show that breakup KIBRA-PKMzeta binding erases old memories. Previous work had shown that random increases in PKMzeta in the brain improved weak or attenuated memories, which was mysterious because it should have done the opposite by acting at random locations, but persistent synaptic labeling by KIBRA explains why the extra PKMzeta improved memory, by acting only at sites labeled by KIBRA.
“The persistent synaptic tagging mechanism explains for the first time these findings that are clinically relevant to neurological and psychiatric memory disorders,” observes Fenton, who is also on the faculty of the Neuroscience Institute at NYU Langone Medical Center.
The study authors point out that this research confirms a concept introduced in 1984 by Francis Crick. Sacktor and Fenton point out that his hypothesis to explain the brain’s role in storing memory despite constant cellular and molecular changes is a “Ship of Theseus” mechanism, borrowed from a philosophical argument from Greek mythology in which new planks replace old ones to keep the Ship of Theseus in good condition for years.
“The persistent synaptic tagging mechanism we discovered is analogous to how new planks replace old ones to maintain the Ship of Theseus for generations, and allows memories to last for years even when the proteins that maintain the memory are replaced,” Sacktor says. “Francis Crick intuited this Ship of Theseus mechanism, even predicting the role of a protein kinase. But it took 40 years to discover that the components are KIBRA and PKMzeta and to understand the mechanism of their interaction.”
Reference: “KIBRA anchors the action of the PKM? maintains the persistence of memory” by Panayiotis Tsokas, Changchi Hsieh, Rafael E. Flores-Obando, Matteo Bernabo, Andrew Tcherepanov, A. Iván Hernández, Christian Thomas, Peter J. Bergold, James E. Cottrell, Joachim Kremerskothen, Harel Z . Shouval, Karim Nader, André A. Fenton and Todd C. Sacktor, June 26, 2024, Scientific progress.
DOI: 10.1126/sciadv.adl0030
The study also included researchers from McGill University in Canada, University Hospital Münster in Germany and the University of Texas Medical School at Houston.
This work was supported by grants from the
” data-gt-translate-attributes=”({“attribute”:”data-cmtooltip”, “format”:”html”})” tabindex=”0″ role=”link”>National Institutes of Health (R37 MH057068, R01 MH115304, R01 NS105472, R01 MH132204, R01 NS108190), the Natural Sciences and Engineering Research Council of Canada Discovery (203523) and the Garry and Sarah S. Sklar Fund.